Uncategorised
Προγεννητική πατρότητα
- Written by: Hexabit
Η πατρότητα μπορεί να προσδιοριστεί και πριν τη γέννηση του παιδιού από την 11η εβδομάδα της κύησης. Γίνεται με συλλογή αίματος από τη μητέρα, με τη διαδικασία του προγεννητικού ΝΙΡΤ τεστ, και αίμα από τον πατέρα. Από το μητρικό αίμα απομονώνεται το εμβρυϊκό DNA και εξετάζονται 400 γενετικοί τόποι. Οι ίδιοι τόποι εξετάζονται και στο DNA του φερόμενου ως πατέρα. Το αποτέλεσμα επιβεβαιώνει ή αποκλείει τον πατέρα με ακρίβεια 100%.
Η διαδικασία είναι απόλυτα εχέμυθη και συμφωνείται εκ των προτέρων ο τρόπος αποστολής του αποτελέσματος, ο αποδέκτης και η διεύθυνση.
Proderma
- Written by: Hexabit

Preparation of innovative and advanced antimicrobial skin regeneration patches supplemented with extracellular matrix components and stem cells T6YVP-00288 with the co-funding of Greece and European Union
OBJECTIVE
Preparation of innovative and advanced skin regeneration patches with inherent antimicrobial activity supplemented with extracellular matrix components and stem cells.
Today, regeneration and healing of the skin occurs mainly through invasive methods, which are painful, time consuming, costly and stressful for patients. A new trend in the healing of skin wounds is the use of hydrogel-type polymeric patches which maintain a moisture level suitable for tissue regeneration.
However, these patches trap moisture in the skin, which, while it is necessary for tissue growth, is also an agent for the growth of microbes. To address this, antimicrobial drugs have been used but they were found to slow down the regeneration of skin tissue. Thus, the development of innovative polymeric patches whose molecules carry antimicrobial compounds or possess antimicrobial topography are a promising solution to this problem.
BIOHELLENIKA biotechnology company, with years of experience in the stem cell field and research on cellular therapies and modern equipment on its premises, NANOTYPOS O.E. nanoprint lithography research and development company, with extensive know-how in nano- production processes and innovative facilities, and the team of Professor Bikiaris with the necessary experience and the appropriate laboratory equipment for the development of new advanced polymeric materials, are working together to:
- Develop hydrogels with inherent antimicrobial activity by chemically modifying and surface topography.
- Further boost the prepared hydrogels with bioactive peptides and a combination of the latter with ASCs (Human Adipose Stromal Cells) which are essential for skin growth and complete healing of wounds.
- Assess the action of advanced skin regeneration patches.
Project Coordination: BIOHELLENIKA (Dr. George Koliakos, MD)
Principal Investigator: Dr Kokkona Kouzi-Koliakou, MD
Duration: 1/05/2020-27/08/2023

Newsletters
- Written by: Hexabit
ΟΡΟΙ ΧΡΗΣΗΣ ΙΣΤΟΣΕΛΙΔΑΣ
- Written by: Hexabit
Η ιστοσελίδα «biohellenika.gr» [εφεξής «η Ιστοσελίδα»], ιδιοκτησίας της ανώνυμης εταιρείας με την επωνυμία «BIOHELLENIKA ΑΝΩΝΥΜΟΣ ΕΤΑΙΡΙΑ – ΕΤΑΙΡΙΑ ΕΝΤΑΣΗΣ ΓΝΩΣΗΣ – ΜΕΤΑΦΟΡΑΣ ΤΕΧΝΟΓΝΩΣΙΑΣ – ΤΕΧΝΟΛΟΓΙΑΣ – ΒΙΟΤΕΧΝΟΛΟΓΙΑΣ – ΠΑΡΟΧΗΣ ΥΠΗΡΕΣΙΩΝ ΚΑΙ ΣΥΜΒΟΥΛΩΝ» και διακριτικό τίτλο «Biohellenika Α.Ε.», που εδρεύει στην Πυλαία Θεσσαλονίκης, επί της Λεωφόρου Γεωργικής Σχολής αρ. 65, [εφεξής «η Εταιρία»] προσφέρει το περιεχόμενό της στους επισκέπτες της υπό τους παρακάτω αναφερόμενους όρους και προϋποθέσεις.
Βεβαιωθείτε ότι συμφωνείτε με τους παρακάτω όρους και προϋποθέσεις, δεδομένου ότι η περαιτέρω χρήση και η περιήγησή σας στην ιστοσελίδα μας, συνεπάγεται την ρητή και ανεπιφύλακτη συγκατάθεση και συναίνεση σας με αυτούς.
Α. Γενικοί Όροι
1. Οι επισκέπτες της Ιστοσελίδας έχουν την ευθύνη πρόσβασης σε αυτή και βαρύνονται με την τυχόν καταβολή τελών σε τρίτους φορείς που συνδέονται με την πρόσβαση και παραμονή σε αυτή (π.χ. χρεώσεις από παρόχους υπηρεσιών διαδικτύου).
2. Η Εταιρία δεν εγγυάται ότι η Ιστοσελίδα θα είναι διαρκώς προσβάσιμη ούτε ότι οι πληροφορίες που περιέχονται σε αυτή είναι πάντοτε επικαιροποιημένες. Στην Ιστοσελίδα δεν περιλαμβάνονται συμβουλές οποιουδήποτε είδους.
3. Η Ιστοσελίδα και τα επιμέρους στοιχεία αυτής (κείμενα, φωτογραφίες, γραφικά κλπ) προστατεύονται με δικαιώματα πνευματικής ιδιοκτησίας, αποκλειστικός δικαιούχος των οποίων είναι η Εταιρία. Απαγορεύεται οποιαδήποτε αντιγραφή, αναπαραγωγή, παρουσίαση στο κοινό, κατ’ αίτηση διάθεση ή οποιαδήποτε άλλη χρήση του περιεχομένου της Ιστοσελίδας χωρίς την προηγούμενη έγγραφη άδεια της Εταιρίας.
4. Το όνομα χώρου «biohellenika.gr, η φράση «Biohellenika» και το λογότυπο «Biohellenika» αποτελούν ιδιοκτησία της Εταιρίας και προστατεύονται από τις διατάξεις για τα domain names και τα διακριτικά γνωρίσματα αντίστοιχα.
5. Η Ιστοσελίδα πιθανόν να συνδέεται με άλλες μέσω υπερσυνδέσμων. Η Εταιρία, οι νόμιμοι εκπρόσωποί της ή οι εργαζόμενοί της δεν φέρουν καμία ευθύνη για τυχόν προσβολή ή ζημία που θα προκύψει από την επίσκεψη και χρήση των ιστοσελίδων που συνδέονται με τη Ιστοσελίδα. Επισκέπτες που μεταβαίνουν σε συνδεόμενες με την παρούσα ιστοσελίδες, το πράττουν με δική τους αποκλειστική ευθύνη. Ειδικότερα η Εταιρία, οι νόμιμοι εκπρόσωποί της ή οι εργαζόμενοί της δεν φέρουν καμία ευθύνη για τυχόν προσβολή ή ζημία που θα προκύψει από επικοινωνία ή εμπορική συναλλαγή με ιστοσελίδες τρίτων, που συνδέονται μέσω υπερσυνδέσμου με την Ιστοσελίδα.
6. Απαγορεύεται οποιαδήποτε ενέργεια που μπορεί να προκαλέσει ζημιά στην Ιστοσελίδα ή μπορεί να επηρεάσει τη διαθεσιμότητα ή την προσβασιμότητα της Ιστοσελίδας ή είναι με οποιονδήποτε τρόπο παράνομη ή επιβλαβής ή σχετίζεται με παράνομη ή επιβλαβή δραστηριότητα ή σκοπό.
7. Απαγορεύεται η χρήση της Ιστοσελίδας με σκοπό την αντιγραφή, αποθήκευση, μετάδοση ή διανομή οποιουδήποτε υλικού που συνιστά ή συνδέεται με λογισμικό spyware, ιό υπολογιστή, Trojan horse και εν γένει με κάθε είδους κακόβουλο λογισμικό υπολογιστή.
Οι χρήστες της ιστοσελίδας αποδέχονται ότι δεν θα χρησιμοποιούν την ιστοσελίδα της Biohellenika για αποστολή, δημοσίευση, αποστολή με e-mail ή μετάδοση με άλλους τρόπους οποιουδήποτε περιεχομένου είναι παράνομο, βλαβερό, απειλητικό, προσβλητικό, ενοχλητικό, συκοφαντικό, δυσφημιστικό, χυδαίο, άσεμνο, λιβελογραφικό ή που να αποτελεί παραβίαση του απορρήτου κάποιου άλλου, να δείχνει εμπάθεια, ή να εκφράζει φυλετικές, εθνικές ή άλλες διακρίσεις, να δύναται να προκαλέσει βλάβες σε ανήλικους με οποιονδήποτε τρόπο, δεν δικαιούται να μεταδοθεί σύμφωνα με την νομοθεσία ή τις συμβατικές ή διαχειριστικές σχέσεις (όπως εσωτερικές πληροφορίες, ιδιοκτησιακές και εμπιστευτικές πληροφορίες που αποκτήθηκαν ή αποκαλύφθηκαν ως μέρος εργασιακών σχέσεων ή που καλύπτονται σε συμφωνίες εμπιστευτικότητας), να παραβιάζει οποιαδήποτε ευρεσιτεχνία, εμπορικό σήμα, εμπορικό μυστικό, πνευματικά δικαιώματα ή άλλα ιδιοκτησιακά δικαιώματα της Biohellenika και τρίτων, να περιέχει ιούς λογισμικού ή οποιουσδήποτε άλλους κώδικες, αρχεία ή προγράμματα, που έχουν σχεδιαστεί με σκοπό την διακοπή, την πρόκληση βλάβης, την καταστροφή ή τον εξοπλισμό της λειτουργίας οποιουδήποτε λογισμικού ή υλικού υπολογιστών, ηθελημένα ή αθέλητα να παραβαίνει την ισχύουσα ελληνική και κοινοτική νομοθεσία και των διατάξεων αυτής, να δύναται να παρενοχλήσει τρίτους με οποιοδήποτε τρόπο και οποιοδήποτε περιεχόμενο χρησιμοποιείται για συλλογή ή αποθήκευση προσωπικών δεδομένων σχετικά με άλλους χρήστες.
8. Απαγορεύεται το scraping, data mining, data extraction και data harvesting από την Ιστοσελίδα χωρίς την έγγραφη άδεια της Εταιρίας.
9. Η Biohellenika διατηρεί το δικαίωμα να τροποποιεί μονομερώς ή να ανανεώνει τους παρόντες όρους και τις προϋποθέσεις χρήσης του ιστοσελίδας, σύμφωνα με τις ανάγκες της και τα συναλλακτικά ήθη.
10 H Biohellenika δεσμεύεται ως προς την ποιότητα, την πληρότητα και την εγκυρότητα των πληροφοριών που παρατίθενται στην ιστοσελίδα της www.biohellenika.gr όσον αφορά τα ακριβή στοιχεία που εκτίθενται υπό την επιφύλαξη τυχόν τεχνικών ή τυπογραφικών λαθών, που δεν μπορούν να προβλεφθούν ή που έχουν προκύψει ακούσια ή λόγω διακοπών λειτουργίας της ιστοσελίδας από λόγους ανωτέρας βίας.
11. Σε καμία περίπτωση η Biohellenika δεν φέρει ευθύνη για τυχόν απαιτήσεις νομικής ή αστικής ή/και ποινικής φύσης ούτε για τυχόν ζημία (θετική, ειδική ή αποθετική, η οποία ενδεικτικά και όχι περιοριστικά, διαζευκτικά ή/και σωρευτικά συνίσταται σε απώλεια κερδών, δεδομένων, διαφυγόντων κερδών, χρηματικής ικανοποίησης, κ.τ.λ.) από επισκέπτες του δικτυακού τόπου ή τρίτους από αιτία που έχει σχέση με τη λειτουργία ή μη ή/και τη χρήση του δικτυακού τόπου ή/και σε αδυναμία παροχής υπηρεσιών ή/και πληροφοριών που διατίθενται από αυτόν ή/και από τυχόν μη επιτρεπόμενες παρεμβάσεις τρίτων σε προϊόντα ή/και υπηρεσίες ή/και πληροφορίες που διατίθενται μέσω αυτού.
12. Αυτή η ιστοσελίδα αποτελεί την επίσημη ιστοσελίδα της Biohellenika.. To πλήρες περιεχόμενο της ιστοσελίδας, όπως αυτό διαμορφώνεται κάθε φορά, συμπεριλαμβανομένων εικόνων, γραφικών, φωτογραφιών, σχεδίων, κειμένων, παρεχόμενων υπηρεσιών και προϊόντων, αποτελούν πνευματική ιδιοκτησία της Biohellenika και προστατεύονται κατά τις σχετικές διατάξεις του ελληνικού δικαίου, του ευρωπαϊκού δικαίου και των διεθνών συμβάσεων. Απαγορεύεται οποιαδήποτε αντιγραφή, αναλογική / ψηφιακή εγγραφή και μηχανική αναπαραγωγή, διανομή, μεταφορά, downloading, μεταποίηση, μεταπώληση, δημιουργία παράγωγης εργασίας ή παραπλάνηση του κοινού σχετικά με τον πραγματικό πάροχο του Περιεχομένου του διαδικτυακού αυτού τόπου. Τυχόν αναπαραγωγή, επανέκδοση, φόρτωση, ανακοίνωση, διάδοση ή μετάδοση ή οποιαδήποτε άλλη χρήση του Περιεχομένου με οποιοδήποτε τρόπο ή μέσο για εμπορικούς ή άλλους σκοπούς επιτρέπεται μόνο κατόπιν προηγούμενης γραπτής άδειας της Biohellenika ή οιουδήποτε άλλου νόμιμου δικαιούχου των ανωτέρω πνευματικών δικαιωμάτων. Τα ονόματα, εικόνες, λογότυπα και διακριτικά γνωρίσματα που αντιπροσωπεύουν την Biohellenika καθώς και τα προϊόντα ή τις υπηρεσίες της, είναι αποκλειστικά σήματα και διακριτικά γνωρίσματα της Biohellenika ή/και του ή/και των άνω τρίτων μερών και προστατεύονται από τους ελληνικούς, κοινοτικούς και διεθνείς νόμους περί εμπορικών σημάτων και βιομηχανικής και πνευματικής ιδιοκτησίας. Σε κάθε περίπτωση η εμφάνιση και έκθεσή τους στην ιστοσελίδα και στο ηλεκτρονικό κατάστημα της Biohellenika δεν θα πρέπει κατά κανένα τρόπο να εκληφθεί ως μεταβίβαση ή εκχώρηση άδειας ή δικαιώματος χρήσης τους.
13. Η Biohellenika λαμβάνοντας υπόψη τη σημασία του θέματος της ασφαλείας των Προσωπικών Δεδομένων των χρηστών, λαμβάνει όλα τα απαραίτητα μέτρα, με τις πιο σύγχρονες και προηγμένες μεθόδους, ώστε να εξασφαλίζεται η μέγιστη δυνατή ασφάλειά τους. Όλες οι πληροφορίες, οι οποίες σχετίζονται με τα προσωπικά τους στοιχεία και τις συναλλαγές τους, είναι ασφαλείς και απόρρητες.
14 Κατά την επίσκεψή σας στην ιστοσελίδα μας για να ενημερωθείτε, αλλά και για να διασφαλισθεί η δυνατότητα επικοινωνίας μαζί σας ώστε να σας ενημερώνουμε για τις νέες υπηρεσίες και τα νέα προϊόντα μας, είναι πιθανό να σας ζητηθεί να δηλώσετε στοιχεία που σας αφορούν (όνομα, ηλεκτρονική διεύθυνση, τηλέφωνο, κ.τ.λ.). Τα τυχόν προσωπικά δεδομένα που δηλώνετε προορίζονται αποκλειστικά και μόνο για τη διασφάλιση της λειτουργίας της αντίστοιχης υπηρεσίας και δεν επιτρέπεται να χρησιμοποιηθούν από οποιονδήποτε τρίτο, χωρίς να τηρηθούν οι διατάξεις των σχετικών νόμων για την προστασία από επεξεργασία δεδομένων προσωπικού χαρακτήρα, όπως αυτός ισχύει κάθε φορά. Τα τηρούμενα στοιχεία του αρχείου δύνανται να κοινοποιηθούν στις αρμόδιες δικαστικές, αστυνομικές και άλλες διοικητικές αρχές κατόπιν νόμιμου αιτήματός τους και σύμφωνα με τις κάθε φορά ισχύουσες νομοθετικές διατάξεις. Ο Πελάτης /Χρήστης έχει, μέσα στα πλαίσια της νομοθεσίας περί απορρήτου των τηλεπικοινωνιών, τα δικαιώματα ενημέρωσης και αντίρρησης που προβλέπουν τα άρθρα 11 έως 13 του ν. 2472/1997.

Ατομική κρέμα προσώπου εμπλουτισμένη με PRP
- Written by: Dr. Kouzi
- Ατομική κρέμα προσώπου με PRP
H my cream δημιουργήθηκε από την επιστημονική ομάδα της Biohellenika με σκοπό να θέσει τις υπηρεσίες της βιοτεχνολογίας στην καθημερινή φροντίδα του δέρματος. Η my cream περιέχει τη βάση της, η οποία ενισχύεται με τα λιποσώματα και τους αυξητικούς παράγοντες που περιέχονται στο PRP (Platelet Rich Plasma).
Τα λιποσώματα αναμειγνυόμενα με το PRP προσφέρουν βαθύτερη και ταχύτερη ενυδάτωση της επιδερμίδας, οδηγούν τους αυξητικούς παράγοντες σε βαθύτερα στρώματα του δέρματος προσφέροντας ολική ανάπλαση με φυσικό, ανώδυνο και μη επεμβατικό τρόπο.
Αναζωογονείστε το πρόσωπο σας με την ατομική σας κρέμα και το PRP
Η κρέμα είναι εξατομικευμένη και αποτελεί πρωτοπορία της Biohellenika. Δημιουργείται στο εργαστήριο ξεχωριστά για τον καθένα και χρησιμοποιείται αποκλειστικά από τον δότη του PRP, λειτουργεί δε σαν ανώδυνη μεσοθεραπεία.
Η my cream χρησιμοποιείται ως μέσον αντιγήρανσης στο πρόσωπο, στο λαιμό και στα χέρια, αυξάνει το τοπικό κολλαγόνο, ελαττώνει τις ρυτίδες, διατηρεί τις λεπτές γραμμές και προσφέρει υγιές και νεανικό δέρμα.
Απλώνεται τις νυχτερινές ώρες σε καθαρό δέρμα με απαλό μασάζ σε όλο το πρόσωπο. Για την αποτελεσματικότερη χρήση της, η my cream φυλάσσεται στο ψυγείο και χρησιμοποιείται μέσα σε ένα μήνα από τη δημιουργία της.
Platelet Rich Plasma (PRP)

Το PRP είναι ένα αυτόλογο παράγωγο που δημιουργείται από το αίμα μετά από κατάλληλη επεξεργασία και περιέχει τους αυξητικούς παράγοντες των αιμοπεταλίων, οι οποίοι προάγουν τον κυτταρικό πολλαπλασιασμό των ινοβλαστών της επιδερμίδας, προστατεύουν το δέρμα από τη φωτογήρανση και προσφέρουν τη μέγιστη δυνατή ανάπλαση, φυσική λάμψη και νεανική εμφάνιση του προσώπου, του λαιμού και των χεριών.
Χρησιμοποιούνται για:
- Ολική αποκατάσταση του προσώπου (περιλαμβανομένων των ρυτίδων του ματιού, του μεσοφρίου, του μετώπου, των ρινοχειλικών), του λαιμού και του θώρακα
- Διορθώνουν τις ουλές της ακμής
- Διορθώνουν τυχόν βλάβες από τη χρήση του laser
Οφέλη από τη χρήση της my cream και του PRP:
- Αναζωογονούν και διεγείρουν τους τοπικούς ινοβλάστες στην περιοχή όπου εφαρμόζονται.
- Επιταχύνουν τη διαδικασία της αναγέννησης και της επούλωσης
- Λειτουργούν συνεργικά με τα βλαστικά κύτταρα
- Η εφαρμογή τους είναι ανώδυνη, απλή και γρήγορη, δεν προκαλούν τραύμα.
- Λειτουργούν σαν μεσοθεραπεία και προσφέρουν φυσικό αποτέλεσμα, χωρίς κίνδυνο ασυμμετρίας ή παραμόρφωσης
- Το αποτέλεσμα που προσφέρουν είναι μακράς διάρκειας
Σε τί διαφέρει το PRP της Biohellenika από τα κυκλοφορούντα στην αγορά με τη μορφή κιτ;
Το PRP παρασκευάζεται από το επιστημονικό προσωπικό της Biohellenika εξατομικευμένα και έχει δοκιμαστεί σε μεγάλη ομάδα ασθενών με συγκριτικά καλύτερα αποτελέσματα, σε σχέση με τα αποτελέσματα των κιτ που κυκλοφορούν στην αγορά.
Ο διαχωρισμός των αιμοπεταλίων από τα υπόλοιπα έμμορφα στοιχεία του αίματος που επιτυγχάνεται με φυγοκέντρηση δεν σημαίνει αυτόματα και τη δημιουργία του PRP. Απαιτείται ένα δεύτερο στάδιο ενεργοποίησης των αιμοπεταλίων πριν την χορήγηση τους. Οι αυξητικοί παράγοντες των αιμοπεταλίων φυσιολογικά βρίσκονται στο εσωτερικό τους και για να δράσουν θα πρέπει απελευθερωθούν στο περιβάλλον.
Το προϊόν PRP παρασκευάζεται με ειδική επεξεργασία 20ml περιφερικού αίματος και μπορεί να χρησιμοποιείται άμεσα ή να καταψύχεται και να χρησιμοποιείται αργότερα.
Stem Cells History
- Written by: Hexabit

Stem cells history
1988: The first transplantation of stem cells is performed in France in a 5 year old boy that was suffering from Fanconi syndrome (aplastic type anemia and deafness). Stem cells from the boy’s sister were used that was born for this purpose. Until today, 20 years after, the boy, an adult today, is healthy.
1991: The University of Cincinnati in United States reported two cases of successful allogeneic transplantation of stem cells from the umbilical cord blood of unrelated histocompatible donors in children with chronic myelogenic leukemia. These transplantations established the treatment of diseases that traditionally have been used grafts from bone marrow with the use of stem cells from the umbilical cord
1992: The first public and private stem cell cryobanks is established in the United States and the first private storage by freezing the stem cells of the umbilical cord of the child one of its founders is performed.
1995: In the medical journal Lancet, Wagner, a great researcher in the field of stem cells transplantations and one of his coworkers reported for the first time an extensive study of the transplantation of stem cells of the umbilical cord in related recipients. The results of the research show that the survival and the successful implantation of the umbilical cord blood grafts are better between relatives, instead of use compatible unrelated donors of bone marrow. Thus stem cells of the umbilical cord blood are considered to have equal importance to the therapeutic applications as the stem cells derived from bone marrow.
1997: The results of another important research are reported, according to which the percentage of survival of leukemia patients that were administered stem cells from related donors were 63%, while from non-related the percentage of survival was only 23%.
1998: The first autologous transplantation of stem cells derived from the umbilical cord blood is performed. (child’s own stem cells were used). The history of the transplantation is as follows: After the fact that a son of a family from Brazil presented leukemia the parents decided – to their great advantage – to store the stem cells of their next child. The second child eventually presented neuroblastoma, a malignant tumor of the neural system that was treated successfully with the transplantation of his own stem cells that the parents had stored for a possible relapse of the leukemia of the first child.
2001: The first study concerning the transplantation of stem cells of the umbilical cord in adults is published according to which the 90% of the grafts were implanted to the patient. In some cases two units of cord blood have been used.
2003: More that 3000 transplantation of stem cells in a worldwide level have been performed the last two years.
2004: Gesine Koegler and her collaborators announced in the scientific journal, Journal of Experimental Medicine, that the blood of the umbilical cord contains apart from hematopoietic stem cells also pluripotent stem cells, capable to transform into other cells of the human body. This fact gives a new perspective in the research and applications of the umbilical cord and makes the private storage important for the use in regenerative medicine.
2006: The European Union expands the use of stem cells of the umbilical cord blood to the therapy of malignancies and autoimmune diseases. So cord blood can be used as an alternative to bone marrow for these diseases.
Biohellenika is established in Greece, the biggest company of stem cell storage.
2007: The first successful autologous transplantation of cord blood to a child that was suffering from acute lymphoblastic leukemia in 2003 at the age of 3, and the parents had stored the stem cells in a private bank. Four years after the transplantation (2007), the child is alive and in good health. The stem cells were injected immediately and the danger of rejection was minor, as the stem cells were derived from the child himself.
2008: The results of clinical trials for the applications in even more diseases establish the umbilical cord blood stem cell as an invaluable biologic material. Biohellenika established the method to cryopreserve stem cells from the pulp of deciduous teeth and offers a second chance to the parents and adults to cryopreserve this type of stem cells.
2009: Biohellenika released one graft of umbilical cord blood from its labs that was transferred to USA (Duke University) and was administered to a child with cerebral palsy. This child is participating in a worldwide trial among 160 other children and the results of the treatment are very promising, as were recently announced. A second child with cerebral palsy is going to reinfused with its own umbilical cord blood stem cells that its parents have the foresight to cryopreserve in Biohellenika and will travel to the Duke University.
Procedure
- Written by: Dr. Kouzi
Offices
For the parents service the offices of Biohellenika are open in Thessaloniki in ZEDA building daily from 9 am till 9 pm and on Saturday from 9 am till 4 pm. Also, an office at 137 Tsimiski street on the 6th floor, is open daily 5-9pm and the rest of the day if it is asked so. Tel 2310 474282-4
For the South Greece families’ service, offices of the company are open in Athens, Archelaou 28A Pagrati. Furthermore, for the service of the families who live in Thessaly offices are open in the central square of Larissa, 23 M. Alexandrou str., on the 6th floor, tel 2410-535603, office in Patras Kos and Panepistimiou 3, thl 2610437436, Iraklion Amalthias and Katexaki tel 6970803497. Ioannina Park of Technology, Ioannina tel 2651097667, 6970267540, 6949441906
Information
The parents can be informed for the provided service, the potentiality of its application and can discuss the need of the service use based on the medical history of the family. The information is given by responsible scientists of the company, or after an appointment, by a medical professor of the Medical School.
The parents, having an appointment, can also visit the labs of the company and have a tour by a special scientist.
Receipt of the collection package
The parents receive by our offices without any charge the bag for the collection of the umbilical cord blood (collection bag) as well as the documents accompanying it. The parents, having no obligation, receive the contract in order to study it.
The collection bag for the umbilical cord blood is followed by the strictest national specifications so that the quality of the sample is secured as long as it is transported.
The collection bag for the umbilical cord blood, the documents which accompany it and the contract may be delivered at home or they can be posted to you with company’s expenses.
Financial agreement and signing of the contract
The parents are to pay or to agree on the way they want to pay and to sign the contract only after the successful cryo-preservation and the fulfilling of the quality control.
The Biohellenika contract has been done to protect the children and the parents from any possible danger of the sample loss and it improves itself all the time for the clients’ benefit. Every improvement and additional right or ensuring concerns retroactively all the prior clients.
Biohellenika gives priority to the quality and the best service of its clients. For this reason it makes sure, as far as it is possible, to keep the price of the service affordable for the family. Taking that under consideration we have made agreements with banks, to make it easy for the parents to pay in full with 12 installments without interest.
The sample’s collection
Biohellenika has achieved the minimum collection time for the blood samples in Thessaloniki, Athens and Larisssa 1 hour and for the rest of Greece 7 hours. Biohellenika respecting the choice and the the parents to shield their children’s health has its labs in daily function during holidays and furthermore, in two rolling shifts.
In Thessaloniki, Athens, Patra, Larissa, Heraklio and Chania the collection bag is taken immediately from the obstetric clinics by a representative of our company. In other cities the company collects the bags, with its responsibility, from the drivers of the public transport means and informs the parents at once for the collection of the sample and the safe arrival to our labs. It is also parents’ choice the use of a specialized company. For the Aegean islands, the company is cooperating with two transportation companies which are specialized on the transportation of medical samples.
A study of the Scientific Team of Biohellenika which refers to the effect of the conditions and the time of the umbilical cord blood transportation from the obstetric clinic to the lab, concerning the viability of the stem cells, is about to be published in the international scientific Journal Transfusion, official Journal of the American Association of Blood Banks (AABB). In this publication it is proved that the within 12-hours cryo-preservation and until then the preservation stay at 4˚C, constitute the conditions which assure excellent viability of the stem cells.
Placenta-Cord Blood Unit
- Written by: Dr. Koliakos

Umbilical cord blood: (Classic puncture of umbilical cord blood vessels)
The collection of blood is made by your obstetrician immediately after labor. The proper collection of blood is very important for the quality and quantity of the cell.
The process is simple, takes about 5 minutes and is completely safe for the child and for the mother, (as it occurs after birth). Parents must take on time the proper kit of Biohellenika, which must remain in perfect condition until transferring to the labour room on the day of delivery. The kit is packaged in isothermal condition with a registration of temperature to keep the cord blood at a stable temperature.
Cord blood is collected in a special sterile bag and sent to laboratories of Biohellenika, where there is going to be done all the controls and the cryo – preservation of stem cells. We have observed that the collection of stem cells is different in every birth and for this reason it is absolutely necessary to collect the maximum amount of blood. In case of twins-multiple pregnancy, the volume collected from each child is smaller, but this does not cause problems for further use. All data so far, indicate that the age, maternal ethnicity, weight taken by mother during the pregnancy and sex of the child, did not play any role, positive or negative, on the successful collection of cord blood. The things that seems to have result in receiving a higher volume of blood, is the child’s weight at birth and the completion time of pregnancy. Usually, as larger volume of blood collected, as higher the final number of stem cell frozen. Forthwith, the specific isolation method used by Biohellenika, can process small volumes samples. So, Biohellenika’s final acceptance test of the sample is the number of cells in the sample and not the original volume of it.
Quality Control
- Written by: Dr. Kouzi
Cytological control
All photographs are provided by the scientific team of Biohellenika.
Τhree types of measurements are performed before and after the procedure of stem cells separation
1. White and red blood cells count with the certified for clinical use Beckman Coulter (CE-IVD)
2. Viability and number of haematopoietic stem cells (HPC) CD45dimCD34+, according to the ISHAGE protocol by the flow cytometer Epics XM Beckman Coulter
3. Culture of a small number of haematopoietic cells in a specific medium for colony forming BFU-E CFU-G and CFU-GM.
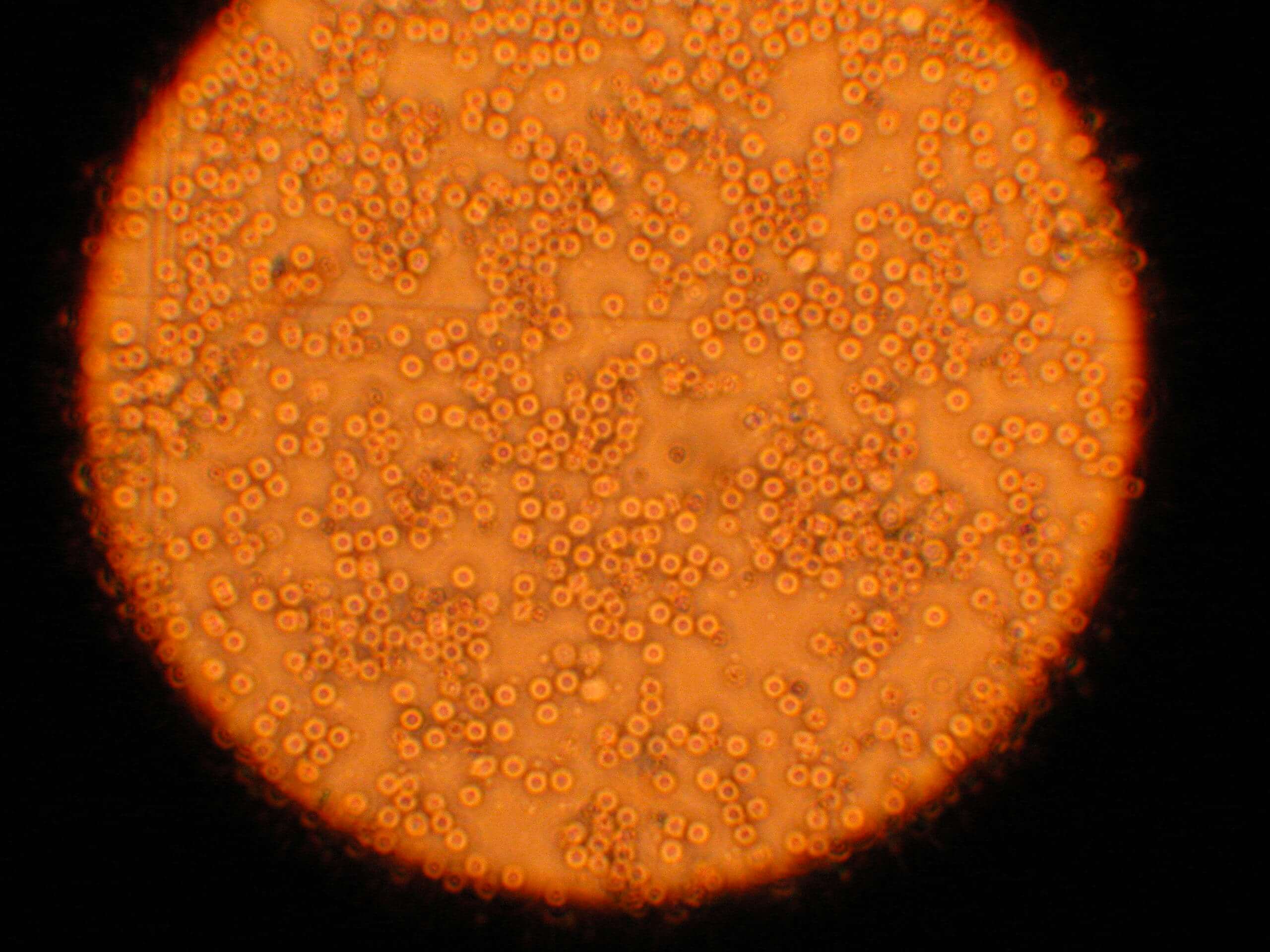
Cell Types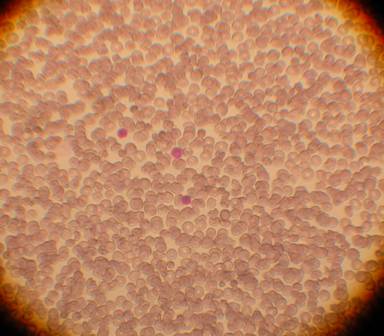
Image 1. The total blood as first coming in the lab, before processing. We can see three nucleated cells and the rest red blood cells. Red blood cells are not stem cells. Stem cells are included in the nucleated population
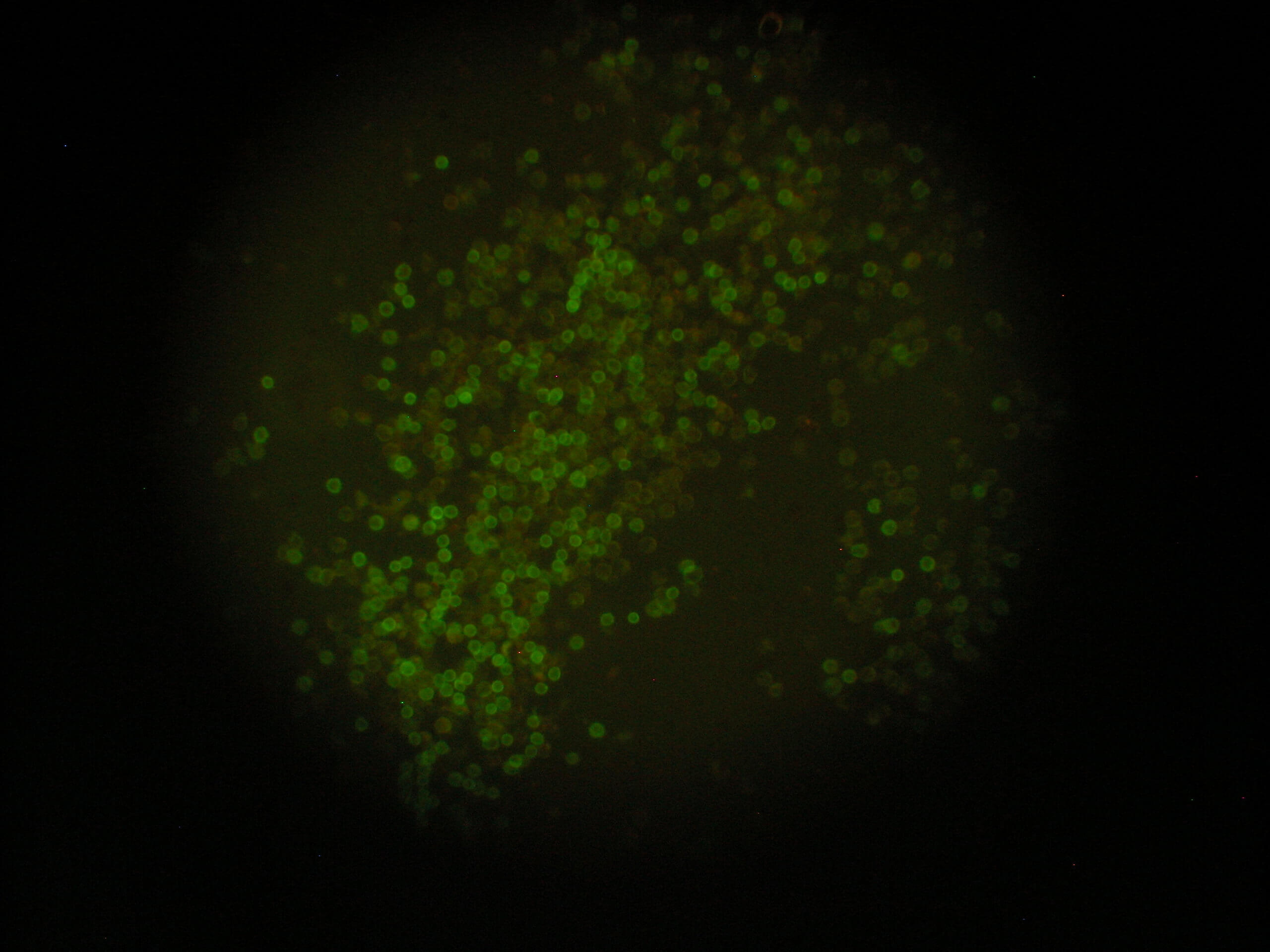
The population of leukocytes in the umbilical cord blood contains 2 types of cells: the haemopoietic population of CD45dim/ CD34+ cells and the population of multipotent CD45-/CD34 cells. VSELs are included in this population. Both populations show great differences in their morphology and their development. The haemopoietic population comprises of more mature cells which constitute the population that will be transformed to haemopoietic cells after transplantation (Images 2 & 3).
|
|
|
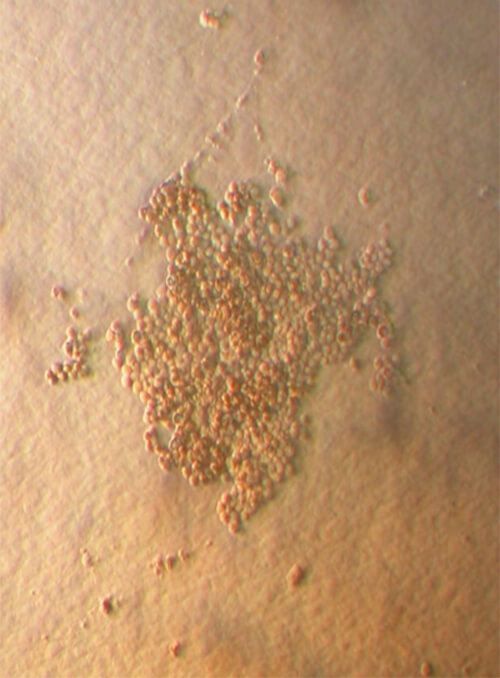
| Image 2 Homogenic population of nucleated cells of the umbilical cord blood after enrichment. Note the total absence of red blood cells after the completion of Biohellenika’s isolation protocol |
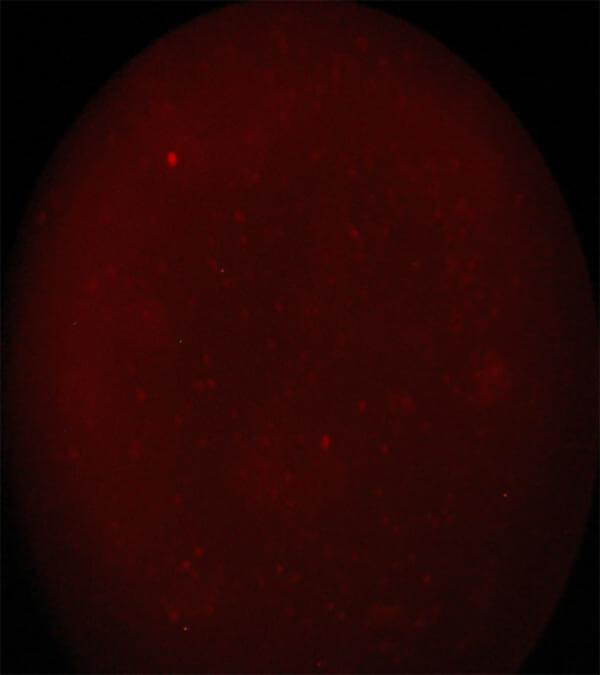
Image 3 CD 45+ cells. Immunofluorescence method using a reverse microscope Zeiss AXIONVERT 40 CFL |
The population of multipotent cells consists of spindle type cells with extensions resembling fibroblasts, and is characterized by the ability to divide 40 times without further differentiation, reaching a population of 1,015 cells without karyotype changes (Gogler et al, 2004, 200: 123-135). This population is a subject of intensive research all over the world and today this type of cells can differentiate in neurons, osteocytes, chondrocytes, fat, cardiac cells, muscular and hepatic cells (See Image 4).
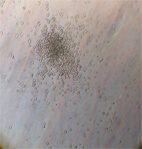
Image 4
Appearance of multipotent stem cells of fibroblast type
in umbilical cord blood after culture for 15 days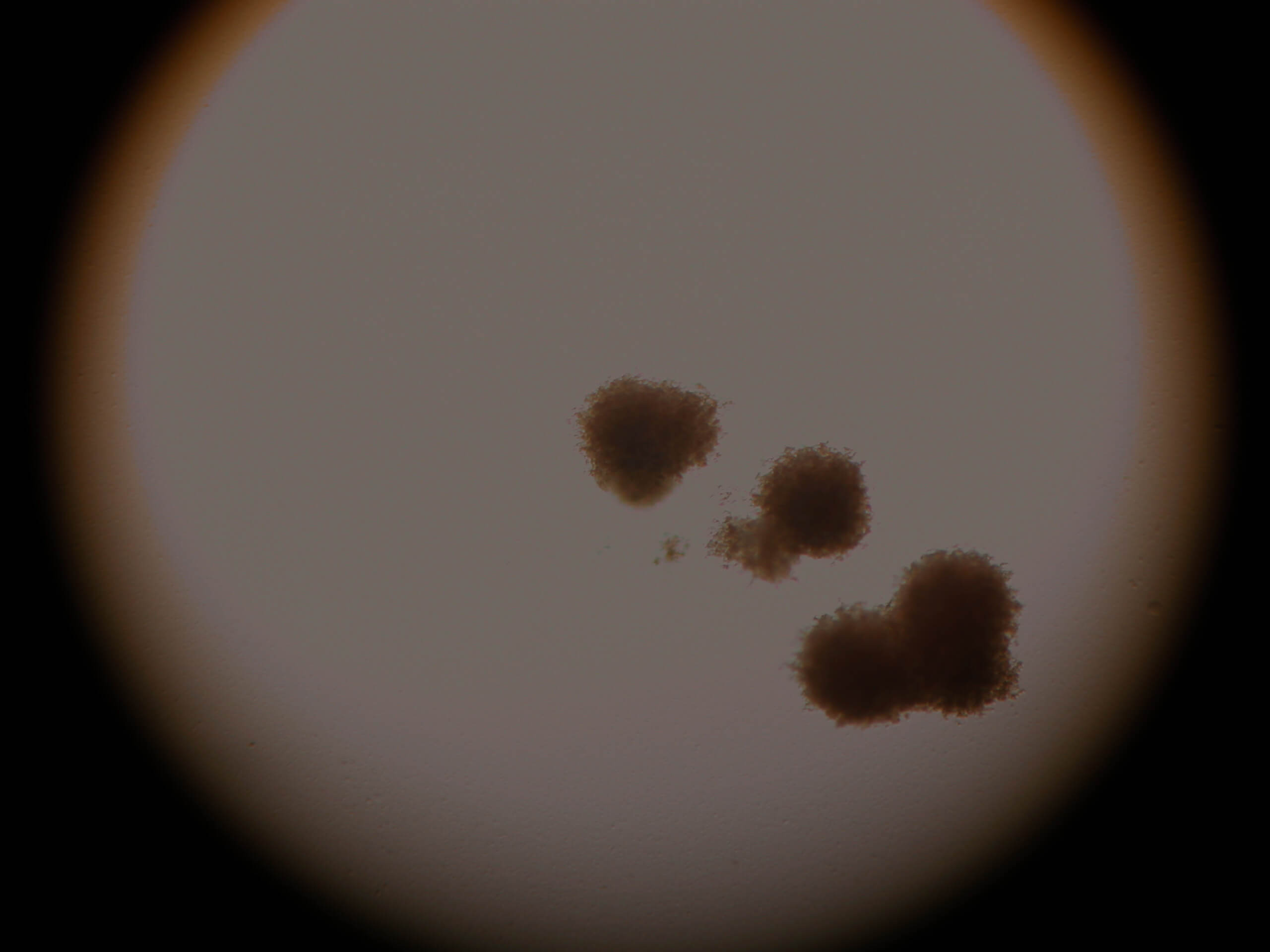
Image 5. CFU of red blood cells. Stem cells maintain all the biological abilities.
 |
 |
| Image 6 | Image 7 |
Images 6-7. CFU of neutrofil-granulocytes-macrophages
Therefore, the CD34+ proliferation for the increase the number of stem cells for haemopeisis and to treat leukaemia is not in use. Today small molecules (like nicotinamide) are tested for CD34+ expantion succesfully. The number of stem cells required for the treatment of leukaemias depends on the body weight of the recipient. The number of nucleated cells from umbilical cord blood reaching 1.73x107 /Kg body weight and the number of CD34+ cells reaching 2.7x105 /Kg body weight, have been administered successfully to patients with leukaemias (Ren et al 2001, Zhonghua, 22: 621, Cilley et al 2004, Bone Marrow Transplantation, 33: 161-4, Magro et al 2006, Hematologica, 91: 640-8). The number of stem cells is proportional to the total volume of collected blood. However, due to the presence of multipotent cells that allow proliferation, even small quantities of blood can nowadays be cryopreserved.
Virology control
Virology tests l for hepatitis B,C,HIV and CMV in the umbilical cord blood with the method RT.PCR.
RT.PCR can detect immediately the virus load in child’s blood and relieves mothers from continuous examination during and after pregnancy. Recently Biohellenika was equiped with Compass instrument by Roche for the direct virus detection in the babys umbilical cord blood.
Bacteriology control
Bacterial aerobious and anaerobious control in three certified for clinical use (CE-IVD) Bactec systems (Becton Dickinson) of total capacity of 290 samples. These systems are characterized by their great sensitivity and reliability of the results.
3-12% of umbilical cord blood units worldwide are considered unsuitable due to contamination. According to our experience in private obstetrics clinics is nearly (0.5-1%) due to special care and skill of obstetricians.
In case of positive bacteriological control, the sample might not be suitable for use but only after the bacterial characterization and the antibiotics and therefore is cryo-preserved for free.
Results of the quality check
The parents receive the medical results of the quality control signed by the doctor in charge (university professor).
All the above mentioned quality methods are accredited by AABB and the Hellenic Accreditation System according to 410, 8/2/2008 published document.
Bibliography - Biohellenika
- Written by: Dr. Koliakos

Bibliography
• Ljungman et al, Bone Marrow Transplant 2006, 37: 439-49
• Strauer et al, J Am Coll Cardiol, 2005, 46: 1659-61
• Sanberg et al, Ann NY Acad Sci 2006, 1049: 67-83
• Ende et al, J Med, 2001, 32: 241-7
• Rocha et al. Transplants of umbilical cοrd blood or bone marrow from unrelated donors in adults with acute leukemia. Ν Engl J Med 2004, 25; 351: 2276-85
• Locatelli et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood 2005, 105; 1: 410-419
• Kato et al. Cord blood transplantation from sibling donors in Japan. Report of the national survey. Int J Hematol 1998, 67: 389-96
• Varadi et al. Human umbilical cord blood for hematopoietic progenitor cells transplantation. Leuk Lymphoma 1995, 20: 51-58
• Kobylka et al. Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation, 1998; 65(9): 1275-1278
• Eapen et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol 2006, 24: 145-151
• Wiley and Kuller. Storage of newborn stem cells for future use. Οbstetrics and Gynecology Vol 1997, 89 (2): 300-303
• Gutman et al. Autologous transplantation followed closely by reduced-intensity allogeneic transplantation as consolidative immunotherapy in advanced lymphoma patients: a feasibility study. Bone Marrow Transplant 2005, 36; 443-51
• Lychtenstein et al, New England Journal of Medicine 2000: 343 (2): 78-85
• Moezz et al. The effect of cryopreservation on clonogenic capacity and in vitro expansion potential of umbilical cord blood progenitor cells. Transplant Proc 2005, 37 (10): 4500-3
• Sartor et l. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products. Bone Marrow Transplant, 2005 36: 199-20
Bone Marrow
- Written by: Dr. Koliakos
 Bone Marrow
Bone Marrow
Biohellenika selects bone marrow stem cells after aspiration of the iliac crest from patients that are undergone coronary by-pass. The cells after isolation in Biohellenika’s labs are injected directly to the cardiac muscle at the periphery of the infarct in the end of the surgery. The procedure is performed due to prior myocardiac infarction or chronic ischemic myocardiopathy. The stem cells usually develop angiogenesis in the next few months and rescue the "hibernating" myocardium. Clinical trials show that stem cells can improve cardiac function shortly after the administration.
Sample Process
- Written by: Dr. Kouzi
Sample Procurement
The technique of double cell separation is applied depleting that way erythrocytes and accomplishing a small final cryopreservation volume which allows better viability and reclamation.
Patient’s protection from the complications of severe haemolysis and the toxic cryoprotectant reagent (DMSO) is accomplished by this procedure and gives the potential of partial freezing and multiple use of the sample.
The cryopreservation of large volume samples results in stem cell’s death during defrozing as during that step, DMSO and hemoglobin must be removed. Large volume cryopreservation demands plastic freezing bags and cryopreservation in liquid nitrogen vapor phase.
Cryopreservation
The cryopreservation is taking place with a validated, digitally adjustable, controlled rate freezing procedure. The procedure, as it is described above, provides gradual transition in lower temperatures and higher cell viability.
Storage
Samples are fully immersed into the liquid nitrogen in a stable temperature of -196˚C, according to the only published protocol which excellent viability results of the stem cells even after 15 years of cryopreservation (Broxmeyer et al 2003, PNAS, 100: 645-650). It is generally accepted that this preservation protocol is the only one which promises unlimited viability time (up to 2000 years) and for this reason it is followed by the biggest private cord blood banks of the world.
On the contrary, public banks, limiting the use of the umbilical cord blood stem cells only for haematopoietic diseases, without substantial financial resources, they store their samples in liquid nitrogen vapour phase as they do not aim in long term preservation.
The preservation places are completely secure, monitored in a 24-hours basis and away from sources of radioisotopes and ionized radiation
Processing takes place in a closed system, without the transplant coming into contact with the environment, with a modification of Rubinstein’s classic protocol, using our own patented processing sac. Only materials approved the Hellenic Drug Organization (Hetastarch 10%) are used and removal of red blood cells is achieved from the total freezing volume by 98%. The recovery of nucleated cells of the transplant after processing is close to 100%.
These results are certified from measurements done by a haematic analyser and with a flow cytometer, of 2006 technology (Beckman Coulter). For the measurement of haemopoietic stem cells from the transplant, we only use the reagent kit (stem Kit TM Beckman Coulter) approved for clinical use by the EU.
Our company uses specially EU approved cryovials (CE), accredited by AABB, that ensure the stable cryopreservation conditions and the theoretical indefinite viability. The cryovials are numbered with a unique code which is also written as a barcode so that it can be read by a computer.
The cryovials are equipped with a special gasket which renders them impenetrable to liquids and are also wrapped in a special protective tube (cryoflex). Therefore the 100% protection of your child’s transplant is ensured.
Freezing before placing the graft in liquid nitrogen takes place gradually with a special digitally operated apparatus. This process ensures the viability of the stem cells after thawing.
The cryopreservation containers contain an automated filling system and the liquid level and temperature of their surface are checked on a 24-hour basis by using a special alarm system as well as TV surveillance.
Quality control of the sample includes: 1) measurement of the number and viability of the stem cells by using the reagent kit (stem Kit TM Beckman Coulter) approved for clinical use by the EU before and after the process and after thawing of a small volume, 2) bacterial check with the sensitive Bactec system and 3) culture for the confirmation of cell colony development.
Depending on the quantity of the cells contained in the final sample volume, it is adjusted to fit in between two to six cryovials. One part of the sample is preserved in one of Biohellenika’s cryopreservation banks in Thessaloniki and the second in the bank in Athens. This increases the chance of survival in the event of a natural disaster and provides the opportunity of secondary use of the initial sample, without thawing and re-freezing.
Wharton’s Jelly
- Written by: Dr. Koliakos
Tissue of umbilical cord (Wharton’s jelly)
In recent years it is discovered that the umbilical cord substance, Wharton’s jelly, contains stem cells that can turn into other cells of the body. These cells are the type of mesenchymal cells and found in tissue surrounding blood vessels of the umbilical cord. The mesenchymal cells are particularly cells and collected by a special method, which separates them from the tissue of the umbilical cord.
The R&D team of Biohellenika has developed the technology and offers to the parents the maximum number of mesenchymal stem cells from the total length of the umbilical cord. This way, exploit the whole amount of mesenchymal stem cells and there is no need to proliferate the cells in the future, which in this case is an additional cost for family and most importantly it changes the biological properties of the cells.
Cell proliferation is not prefered for any type of cell, hematopoietic mesenchymal. International rules require minimum intervention in the cells and cellular proliferation leads to the creation of less primitive cells. The specific method used by Biohellenika was published in the international medical magazine Journal of Biological Research in 2011 and the Journal Transfusion Medicine in 2011 and it is different from cryopreserving whole pieces of umbilical cord.
The cryopreservation of umbilical cord segments without prior isolation of cells, a method that may be used by several banks, in a short time, requires no special skills, does not provide the required number of stem cells and more importantly, does not ensure the viability of stem cells after thawing of the segments.
In order to build such a sample would be required to follow the process of cell proliferation after thawing in the future with extra cost.
The mesenchymal stem cells which are located outside and around the vessels of the umbilical cord, can not be collected either with the classical puncture of the cord or the placenta drainage. The cord is collected in a special sterile bag and transferred immediately to the lab. The cells are separated by enzymatic technique, gradually frozen and stored at cryovials, independently from those of cord blood stem cells.
Attention: The parents that choose to bank mesenchymal cells from the cord, must immediately after the labor transfer the cord to the labs, because the tissue is destroyed after disconnection from the bloodstream.
This service is added to the classic collection from the umbilical cord blood vessel puncture and the placenta drainage, offers the highest number of stem cells that can be collected at birth. Since the stem cells of the umbilical cord-placenta unit is qualitatively superior to any other source, it is ensured by Biohellenika that the child and the family from the moment of birth will have the greatest quantity and highest quality of cells.
By using all these three sources during the birth,we secure three independent collections of cells. And in this case, Biohellenika provides the best scientific and professional service, without considering the cost.
This completes any possible collection of stem cells from the placenta and umbilical cord at birth, ensuring the treatment of blood diseases and regenerative medical applications in patients with large body mass, which under other circumstances could not had the opportunity to keep a sufficient number of stem cells for their treatment.
A second publication of Biohellenikas R&D in Transfusion Medicine in 2011 supported and strengthened the the effectiveness of the methode.
Processing
- Written by: Dr. Koliakos
Sample procurement
The technique of double cell separation is applied depleting that way erythrocytes and accomplishing a small final cryopreservation volume which allows better viability and reclamation.
Patient’s protection from the complications of severe haemolysis and the toxic cryoprotectant reagent (DMSO) is accomplished by this procedure and gives the potential of partial freezing and multiple use of the sample.
The cryopreservation of large volume samples results in stem cell’s death during defrozing as during that step, DMSO and hemoglobin must be removed. Large volume cryopreservation demands plastic freezing bags and cryopreservation in liquid nitrogen vapor phase.
Cryopreservation
The cryopreservation is taking place with a validated, digitally adjustable, controlled rate freezing procedure. The procedure, as it is described above, provides gradual transition in lower temperatures and higher cell viability.
Storage
Samples are fully immersed into the liquid nitrogen in a stable temperature of -196 ˚C, according to the only published protocol which excellent viability results of the stem cells even after 15 years of cryopreservation (Broxmeyeretal 2003, PNAS, 100: 645-650). It is generally accepted that this preservation protocol is the only one which promises unlimited viability time (up to 2000 years) and for this reason it is followed by the biggest private cord blood banks of the world.
On the contrary, public banks, limiting the use of the umbilical cord blood stem cells only for haematopoietic diseases, without substantial financial resources, they store their samples in liquid nitrogen vapour phase as they do not aim in long term preservation.
Biohellenika, like other well known international big cord blood banks, preserves the stem cells in two places. One of two equal parts is preserved at Thessalonikis laboratories and the other in Athens laboratories.
The preservation places are completely secure, monitored in a 24-hours basis and away from sources of radioisotopes and ionized radiation.
The reasons that lay the double preservation is the biggest security in case of natural disaster, the potential of multiple use without de-freezing and re-freezing the initial sample and the immediate disposition of the sample upon request all over Greece.
Placenta
- Written by: Dr. Koliakos
Placenta
Biohellenika exhausting all the possibilities derived by a childs birth proceeds to placenta drainage in order to collect the maximum number of stem cells, besides the classic collection by the puncture of the umbilical cord.
A large number of stem cells still remain inside the placenta after the classic blood collection. For this purpose, the whole placenta is transferred to the laboratory, under specified sterile conditions. The placenta has extended network of vessels where is trapped a large number of stem cells and remain there unused, unless, their collection and procedure follows. Biohellenika using a specific published procedure collects all these cells which are added to the initial classic collection. With this method, a two-fold number of stem cells are ensured, stem cells that otherwise, without this second collection, would be discarded with the placenta.
The larger amount of stem cells is very important as we have the potential to use the sample more times, and to treat leukemia to an overweight adult. Thus, the greater amount of stem cells is cryo-preserved at birth, the greater assurance for the child and its family for their lives.
This special procedure of stem cell collection from the inside of the placenta was awarded at the 15th Pan-Hellenic Transplant Conference, which took place in Thessaloniki in December 2007, was referred to as the most interesting medical publication on internet for one week in February 2008 and was published in the international journal, Transplantation Proceedings 2007, 39 (10): 3380-4. The results were verified for second time with a new publication in international journal Transfusion at March 2011.
Our Services
- Written by: Dr. Kouzi
A. Umbilical cord blood: (Classic puncture of umbilical cord blood vessels)
The collection of blood is made by your obstetrician immediately after labor. The proper collection of blood is very important for the quality and quantity of the cell.
B. Placenta
Biohellenika exhausting all the possibilities derived by a childs birth proceeds to placenta drainage in order to collect the maximum number of stem cells, besides the classic collection by the puncture of the umbilical cord.
C. Tissue of umbilical cord (Wharton’s jelly).
The umbilical cord tissue that surrounds the cord vessels contains mesenchymal cells that are collected with a special procedure from the total length of the umbilical cord, under sterile conditions, in a special sterilized kit.
D. Deciduous teeth
Biohellenika now, gives a second chance to children, who have not cryo-preserved their umbilical cord stem cells at birth, to preserve stem cells from their deciduous teeth that are replaced by the permanent teeth from the age of 5-6 years to 12 years. These cells are in the stage of experimental research for regenerative medicine therapies.
E. Adipose Tissue
You can still preserve stem cells for your future health, as it now known, that adipose tissue is rich in stem cells that are useful in applications of regenerative medicine.
F. Bone Marrow
Biohellenika selects bone marrow stem cells after aspiration of the iliac crest from patients that are undergone coronary by-pass.
Stem Cells main
- Written by: Dr. Kouzi
Stem Cells
Stem cells are primary cells which can be transformed into more mature cells of the organism are precursors of all human cells.
Why to collect stem cells
Umbilical cord blood contains the biggest quantity of stem cells of any other source and the chance of their collection is given only once in a child’s lifetime.
Stem Cells Sources
- Cord Blood
- Placenta
- Cord Body (Whartons jelly)
- Deciduous (first) Teeth
- Adipose Tissue
- Bone Marrow
Current Stem Cell Applications
Methods of cryopreservation
Biohellenika follows a specific certified method. Furthermore Biohellenika uses special material and reagents certified from the relevant organizations (FDA, EC.) IVD and for human use.
Stem Cells History
1988: The first transplantation of stem cells is performed in France in a 5 year old boy that was suffering from Fanconi syndrome (aplastic type anemia and deafness). Stem cells from the boy’s sister were used that was born for this purpose. Until today, 20 years after, the boy, an adult today, is healthy.
Bibliography
Links
This page presents interesting links referring to stem cells or medical articles published in internet related to Biohellenika.
Medical Advisory Center
- Written by: Dr. Kouzi
The doctors of Biohellenika answer to your questions
Medical Advisory Center
In Biohellenika operates a modern Medical Center for parents’ information about the applications of stem cells and guide them according to their family or personal medical records. The center was created in 2009 with the contribution of a specialized team of scientists in the health sector, aiming to promote innovative, effective and safe health services.
The philosophy of the doctors who established and operates Biohellenika’s Medical Advisory center is to inform parents and patients about the therapeutic applications of stem cells, the advantages of family banking and to teach and facilitate the scientific community about these unique services. Prerequisite for this is the constant update, the in-depth knowledge of the worldwide current developments of stem cells and the commitment to providing superior quality services at affordable prices.

Aims of the Medical Advisory Center

The Medical Advisory Center of Biohellenika aims to improve health and to treat diseases. The scientific Head of the Center, Dr. George Koliakos collaborates with specialized teams of doctors and according to the international literature, they are designing clinical trials for the treatment of specific types of diseases. Also, scientific conferences for the prevention, current and future uses of stem cells are organized in the frame of these services.
Dr. Kouzi-Koliakos, Professoranf Head of Embryology-Histology Department, conducts personal meetings with the parents and patients for any medical problem and provides guidance.
The Medical Advisory Center supports parents and its operation is characterized by cooperation and understanding medical problems and their difficulties.