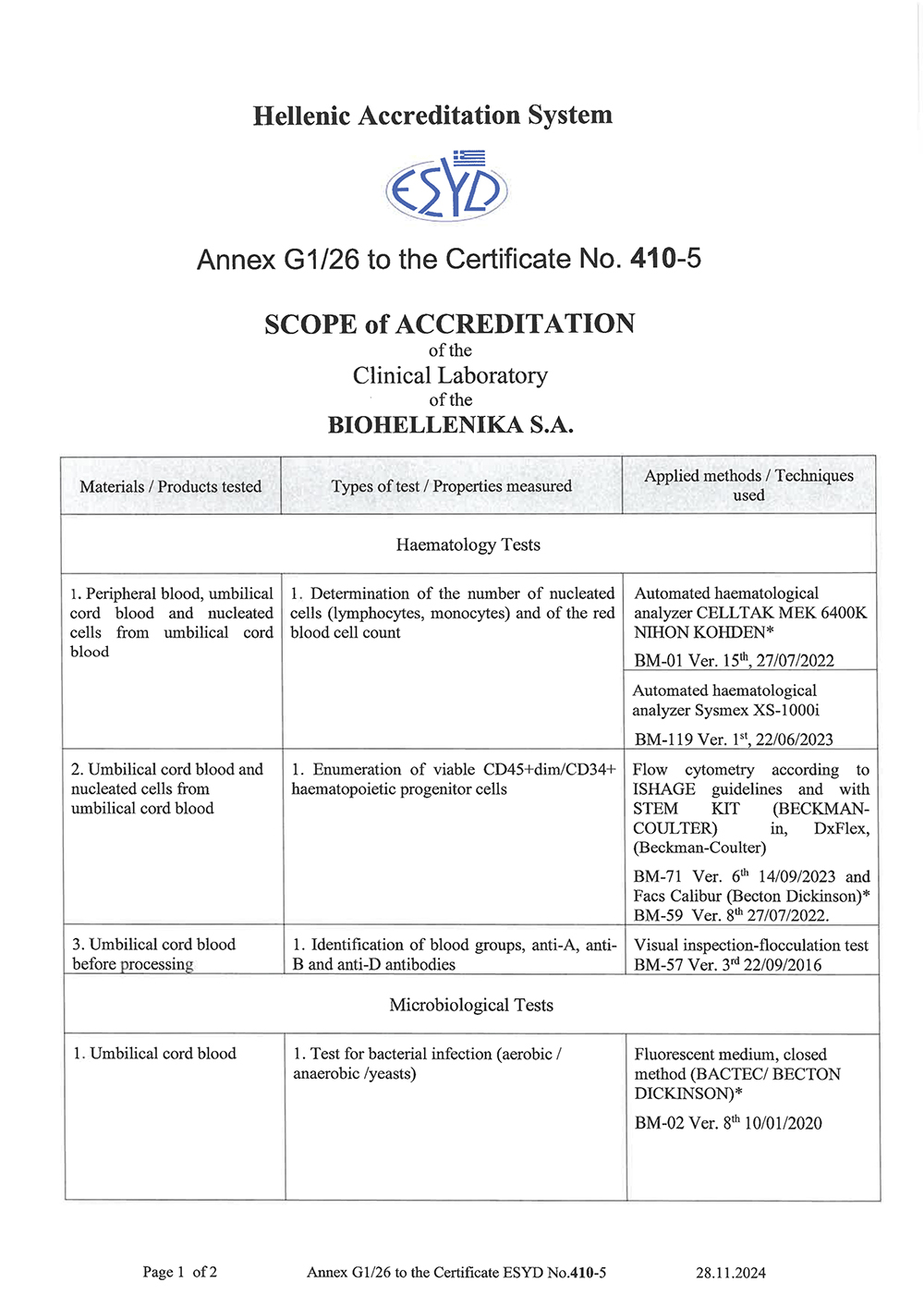
CERTIFICATIONS - ACCREDITATIONS Biohellenikas policy is to gain all the international certifications which are related to the quality for the services provided with the reagents in use and the laboratory equipment. Since the establishment of the company, all procedures are daily controlled and as a result the company succefully received the following certifications and accreditations:
ΑΑΒΒ (AΜΕRΙCAN ASSOCIATION OF BLOOD BANKS)
Biohellenika is accredited by the international recognised organisation AABB (American Association of Blood Banks) since 2010 and renews the accreditation every two years. The present certificate of accreditation is valid until Septeber 2024.
Foundation for the Accreditation of Cellular Therapy